Selecting Laminating Adhesives (by Steven Label)
Surface contact is fundamental to adhesive performance. The ability to get intimate surface contact is a function of the substrate’s surface energy and texture, as detailed below.
Surface Energy
Adhesion is the molecular force of attraction between unlike materials. The strength of attraction is determined by the surface energy of the material. The higher the surface energy, the greater the molecular attraction. The lower the surface energy, the weaker the attractive forces. In other words, on a high surface energy material, the adhesive can flow (or “wet-out”) to assure a stronger bond. High surface energy materials draw the adhesive closer for high bond strength.
The difference is similar to the behavior of water on the finish of a car. On a newly waxed car, water beads up (low surface energy) and on an older finish it wets out (high surface energy).
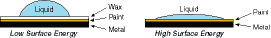
| Surface energy is measured by dynes per centimeter. The dyne level is the actual reading of the critical surface area. The chart below compares the relative surface energy of commonly used substrates. On low surface energy plastics, select an adhesive designed to “flow out” on low surface energy plastics. On high surface energy plastics choose a “harder” adhesive designed for that purpose. |
||
|
|
Texture It’s a common misconception that more adhesive means better adhesion. On smooth surfaces, good surface contact can be achieved with relatively thin layers of adhesive, typically 2 mils. On rough or textured surfaces, more adhesive is required to fill in the hills and valleys, typically 5 mils. |
|